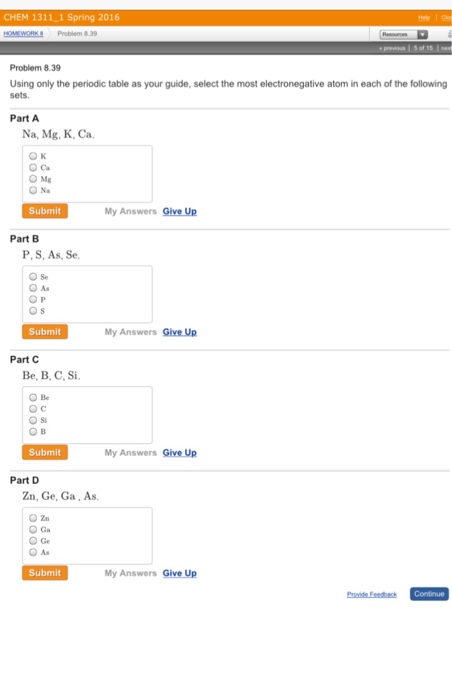
- The Most Electronegative Atom In The Periodic Table Is Quizizz
- The Most Electronegative Atom In A Compound Has A Charge That Is

The elements of the periodic table sorted by electronegativity
click on any element's name for further chemical properties, environmental data or health effects.
This list contains the 118 elements of chemistry.
While there are different scales that you can use such as the Mulliken scale or the Allred-Rochow scale, the one that tends to be most used is the Pauling electronegativity scale. As you can easily see on the image above, the most electronegative atom in the periodic table is fluorine followed immediately by oxygen. 1) the most electronegative atom is generally the one with the largest tendency to pull electrons towards itself. The electronegative of an element is related to ionization energy and electron affinity. In general, an atom with low electronegativity is more metallic (though there are some exceptions) and higher electronegativity is more non-metallic.
| The chemical elements of the periodic chart sorted by: | Electro- negativity | Name chemical element | Symbol | Atomic number |
| - Name alphabetically | 0,7 | Actinium | Ac | 89 |
| - Atomic number | 0,79 | Lanthanum | La | 57 |
| - Symbol | 0,82 | Potassium | K | 19 |
| - Atomic Mass | 0,82 | Strontium | Sr | 38 |
| - Electronegativity | 0,89 | Cerium | Ce | 58 |
| - Density | 0,89 | Thorium | Th | 90 |
| - Melting point | 0,93 | Sodium | Na | 11 |
| - Boiling point | 0,95 | Yttrium | Y | 39 |
| - Vanderwaals radius | 0,98 | Lithium | Li | 3 |
| - Year of discovery | 1 | Potassium | K | 19 |
| - Inventor surname | 1,1 | Praseodymium | Pr | 59 |
| - Elements in earthcrust | 1,1 | Protactinium | Pa | 91 |
| - Elements in human body | 1,12 | Neodymium | Nd | 60 |
| - Covalenz radius | 1,13 | Promethium | Pm | 61 |
| - Ionization energy | 1,14 | Samarium | Sm | 62 |
For chemistry students and teachers: The tabular chart on the right is arranged by electronegativity. The first chemical element is Actinium and the last element is Fluorine. The unity used for the electronegativity is Pauling. Please note that the elements do not show their natural relation towards each other as in the Periodic system. There you can find the metals, semi-conductor(s), non-metal(s), inert noble gas(ses), Halogens, Lanthanoides, Actinoids (rare earth elements) and transition metals. | ||||
| 1,17 | Gadolinium | Gd | 64 | |
| 1,2 | Dysprosium | Dy | 66 | |
| 1,22 | Zirconium | Zr | 40 | |
| 1,22 | Erbium | Er | 68 | |
| 1,23 | Thulium | Tm | 69 | |
| 1,24 | Ytterbium | Yb | 70 | |
| 1,25 | Lutetium | Lu | 71 | |
| 1,27 | Tantalum | Ta | 73 | |
| 1,28 | Curium | Cm | 96 | |
| 1,3 | Tungsten | W | 74 | |
| 1,3 | Uranium | U | 92 | |
| 1,3 | Berkelium | Bk | 97 | |
| 1,3 | Californium | Cf | 98 | |
| 1,3 | Einsteinium | Es | 99 | |
| 1,3 | Fermium | Fm | 100 | |
| 1,3 | Mendelevium | Md | 101 | |
| 1,3 | Nobelium | No | 102 | |
| 1,3 | Lawrencium | Lr | 103 | |
| 1,3 | Rutherfordium | Rf | 104 | |
| 1,3 | Dubnium | Db | 105 | |
| 1,31 | Magnesium | Mg | 12 | |
| 1,33 | Niobium | Nb | 41 | |
| 1,36 | Calcium | Ca | 20 | |
| 1,36 | Americium | Am | 95 | |
| 1,38 | Plutonium | Pu | 94 | |
| 1,5 | Rhenium | Re | 75 | |
| 1,5 | Neptunium | Np | 93 | |
| 1,54 | Scandium | Sc | 21 | |
| 1,55 | Chromium | Cr | 24 | |
| 1,57 | Beryllium | Be | 4 | |
| 1,6 | Molybdenum | Mo | 42 | |
| 1,61 | Aluminum | Al | 13 | |
| 1,62 | Bismuth | Bi | 83 | |
| 1,63 | Titanium | Ti | 22 | |
| 1,65 | Copper | Cu | 29 | |
| 1,66 | Vanadium | V | 23 | |
| 1,69 | Indium | In | 49 | |
| 1,78 | Tin | Sn | 50 | |
| 1,81 | Zinc | Zn | 30 | |
| 1,83 | Manganese | Mn | 25 | |
| 1,88 | Iron | Fe | 26 | |
| 1,9 | Silicon | Si | 14 | |
| 1,9 | Nickel | Ni | 28 | |
| 1,9 | Ruthenium | Ru | 44 | |
| 1,9 | Iridium | Ir | 77 | |
| 1,91 | Cobalt | Co | 27 | |
| 1,93 | Cadmium | Cd | 48 | |
| 1,96 | Antimony | Sb | 51 | |
| 2 | Lead | Pb | 82 | |
| 2 | Radon | Rn | 86 | |
| 2,01 | Gallium | Ga | 31 | |
| 2,02 | Astatine | At | 85 | |
| 2,04 | Boron | B | 5 | |
| 2,05 | Iodine | I | 53 | |
| 2,1 | Xenon | Xe | 54 | |
| 2,16 | Technetium | Tc | 43 | |
| 2,18 | Arsenic | As | 33 | |
| 2,19 | Phosphorus | P | 15 | |
| 2,2 | Hydrogen | H | 1 | |
| 2,2 | Rhodium | Rh | 45 | |
| 2,2 | Silver | Ag | 47 | |
| 2,2 | Platinum | Pt | 78 | |
| 2,2 | Gold | Au | 79 | |
| 2,2 | Francium | Fr | 87 | |
| 2,28 | Palladium | Pd | 46 | |
| 2,28 | Mercury | Hg | 80 | |
| 2,33 | Polonium | Po | 84 | |
| 2,36 | Osmium | Os | 76 | |
| 2,54 | Thallium | Tl | 81 | |
| 2,55 | Carbon | C | 6 | |
| 2,55 | Selenium | Se | 34 | |
| 2,58 | Sulfur | S | 16 | |
| 2,6 | Barium | Ba | 56 | |
| 2,66 | Cesium | Cs | 55 | |
| 2,96 | Krypton | Kr | 36 | |
| 3,04 | Nitrogen | N | 7 | |
| 3,16 | Chlorine | Cl | 17 | |
| 3,44 | Oxygen | O | 8 | |
| 3,98 | Fluorine | F | 9 | |
| Helium | He | 2 | ||
| Neon | Ne | 10 | ||
| Argon | Ar | 18 | ||
| Rubidium | Rb | 37 | ||
| Europium | Eu | 63 | ||
| Terbium | Tb | 65 | ||
| Holmium | Ho | 67 | ||
| Hafnium | Hf | 72 | ||
| Radium | Ra | 88 | ||
| Seaborgium | Sg | 106 | ||
| Bohrium | Bh | 107 | ||
| Hassium | Hs | 108 | ||
| Meitnerium | Mt | 109 | ||
| Darmstadtium | Ds | 110 | ||
| Roentgenium | Rg | 111 | ||
| Ununbium | Uub | 112 | ||
| Ununtrium | Uut | 113 | ||
| Ununquadium | Uuq | 114 | ||
| Ununpentium | Uup | 115 | ||
| Ununhexium | Uuh | 116 | ||
| Ununseptium | Uus | 117 | ||
| Ununoctium | Uuo | 118 |
Click here: for a schematic overview of the periodic table of elements in chart form
Please report any accidental mistake in the above statistics on chemical elements
Lenntech (European Head Office)
Distributieweg 3
2645 EG Delfgauw
The Netherlands
Phone: +31 152 610 900
fax: +31 152 616 289
e-mail: info@lenntech.com
Lenntech USA LLC (Americas)

5975 Sunset Drive
South Miami, FL 33143
USA
Phone: +1 877 453 8095
e-mail: info@lenntech.com
Lenntech DMCC (Middle East)
Level 5 - OFFICE #8-One JLT Tower
Jumeirah Lake Towers
Dubai - U.A.E.
Phone: +971 4 429 5853
e-mail: info@lenntech.com
The Most Electronegative Atom In The Periodic Table Is Quizizz
The Most Electronegative Atom In A Compound Has A Charge That Is
Copyright © 1998-2021 Lenntech B.V. All rights reserved
